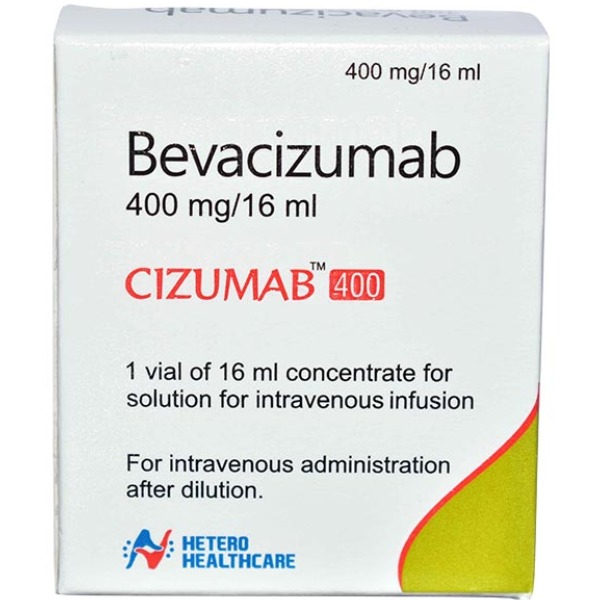
Cizumab 400mg Injection
Dealer, Supplier, Distributor, Exporter, Source, Authorized Distributor, Channel Partner, Vendor
Ernest Pharmaceutical is leading exporter of Cizumab 400mg Injection in Canberra, Melbourne, Sydney, Washington, California, Texas, Riyadh, Jeddah, Buraydah, Tabuk, London, Birmingham, Bristol, Plymouth, Bangkok, Thailand
Cizumab 400mg Injection - Ernest Pharmaceutical
Are you looking for Cizumab 400mg Injection exporter from india?
Contact - https://www.ernestimpex.com
SALT COMPOSITION – Cizumab 400mg Injection (Bevacizumab 400mg)
Storage - Store in a refrigerator (2 - 8°C). Do not freeze.
Product Brochure
Packaging Size - 1 box content 1 vial
Usage/Application - Hospital
Brand - Cizumab 400mg Injection (Bevacizumab 400mg)
Composition - Cizumab 400mg Injection (Bevacizumab 400mg)
Form - Injection
Shelf Life - 24 Months
Marketed By - ERNEST
Country of Origin - Made in India
Minimum order quantity - 10 Box
Uses of Cizumab Solution for Infusion
Cancer of colon and rectum
Non-small cell lung cancer
Cervical cancer
Ovarian cancer
Cancer of fallopian tube
Brain tumor
Kidney cancer
Product introduction
Cizumab 400 Solution for Infusion is an anticancer medication. It is used in the treatment of cancer of colon and rectum, non-small cell lung cancer, kidney cancer, brain tumor, ovarian and cervical cancer. It helps to prevent the growth of new blood vessels that feed tumors and stops tumors from growing.
Cizumab 400 Solution for Infusion is an effective medicine, first-line option when used together with other cancer medicines. It is given as an infusion. That means you get it through a small needle in your vein or through a port, which is a device placed under your skin. The doctor will decide your dose and duration and will check you for signs of an infusion reaction such as high blood pressure and trouble breathing. You keep taking Cizumab 400 Solution for Infusion as long as your disease is controlled and your side effects are manageable. Your doctor will determine whether you should stop taking it or not. You may be advised to check blood pressure and levels of protein in urine while you are taking this medication.
The most common side effects of this medicine include nosebleeds, headache, high blood pressure, rhinitis, protein in urine, change in taste, dry skin, bleeding, lacrimation disorder, back pain, and exfoliative dermatitis. Inform your doctor that you are taking this medication before undergoing any surgical procedure, as the drug has ability to lower the ability of wound healing. Other than this, it also enhances your risk of bleeding thus if you notice any unusual bleeding or bleeding consult with your doctor immediately.
Inform your doctor if you are pregnant, planning pregnancy or breastfeeding. Many other medicines can affect, or be affected by, this medicine so let your healthcare team know all medications you are using.
Side effects of Cizumab Solution for Infusion
Most side effects do not require any medical attention and disappear as your body adjusts to the medicine. Consult your doctor if they persist or if you’re worried about them
Common side effects of Cizumab
Nosebleeds
Headache
High blood pressure
Inflammation of the nose
Protein in urine
Taste change
Dry skin
Hemorrhage
Increased lacrimation
Back pain
Exfoliative dermatitis
www.ernestimpex.com
www.ernestpharmaceuticals.com
Keywords
infusion cancer
cancer medicines
infusion reaction
medicine consult
stop taking
anticancer medication
nose protein
urine change
bleeding consult
unusual bleeding
cervical cancer
youre worried
body adjusts
medical attention
healthcare team
wound healing
surgical procedure
trouble breathing
small needle
stops tumors
feed tumors
origin made
ernest country
refrigerator 2 8c
leading exporter
cizumab solution
blood vessels
side effects
doctor immediately inform
check blood pressure
common side effects
cizumab 400mg injection
high blood pressure
pregnant planning pregnancy
exfoliative dermatitis inform